Atomic hydrogen (deuterium) impurities stabilized in molecular H2 (D2) crystals
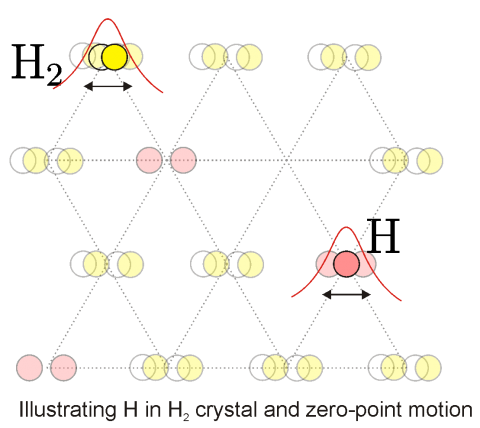
Solid hydrogen is one of the best examples of quantum solids which have large zero-point motion. In case of H2 it is 18% of the lattice constant. Atomic hydrogen and its heavier isotope, deuterium, stabilized in solid H2 and D2 matrices provide a system where a number of fascinating quantum phenomena could be reached.
Already in 1969 Andreev and Lifshitz [1] hypothesized that any impurity in a solid cooled to sufficiently low temperature and having large enough tunnelling probability becomes delocalized and can move freely along the crystal like electrons in conductors. A gas of such impurities or vacancies may exhibit quantum degeneracy phenomena at sufficiently large density, and like the atoms of the ideal gas undergo Bose-Einstein condensation. Chester and Leggett pointed out that phenomenon similar to the superfluidity of 4He may also be reached in its solid phase, so-called “supersolidity”[2,3].
However, if very high concentrations of atoms of the order of a few percents can be reached, the exchange interaction will lead to correlations between their electrons and appearance of electrical conductivity in such system. This would create hydrogen in a metallic state, the “holy grail” of high-pressure physics at zero pressure.
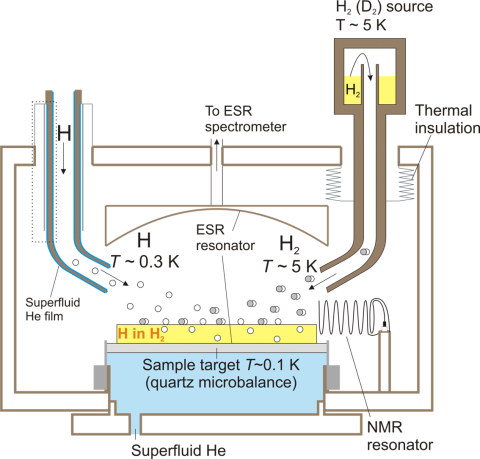
The H,D:H2,D2 samples are prepared by in-situ electron-impact cryogenic dissociation of solid H2 (D2) and studied by magnetic resonance methods (ESR,NMR,ENDOR) in high magnetic field of 4.6T below 1K using a unique cryogenic ESR spectrometer developed in our lab. Recently we have succeeded in reaching record high densities of H atoms, 6×1019cm-3 using exchange quantum reactions D+H2=HD+H and HD+D=H+D2.
At present we are modifying the setup for studies of layered H2,D2 films. A long term goal is to implement atomic and molecular beam epitaxy below 1 K to create samples. It will improve the sample quality and is expected to lead to higher impurity atom densities.
- A.F. Andreev and I.M. Lifshits, Sov. Phys. JETP 29, 1107 (1969)
- G.V. Chester, Phys. Rev. A 2, 256 (1970)
- A. Leggett, Phys. Rev. Lett. 25, 1543.